

Medical tests in the comfort of your own home: Poll shows high interest, uneven use by older adults
From COVID-19 and other infections to cancer and genetic risks, direct-to-consumer tests present new issues for patients, providers & policymakers
Kitchen counters and bathroom sinks across America turned into miniature medical testing labs over the past year, as millions of people swabbed their noses and found out in minutes if they had COVID-19.
Even before the pandemic, many Americans bought tests that had them spit into a tube at home and pop it in the mail, so a company could run tests and alert them to potential health risks lurking in their DNA.
In fact, a new poll shows, 48% of people age 50 to 80 have bought at least one kind of at-home health test, including 32% who had bought COVID-19 tests, 17% who had bought a DNA test, and lower percentages who had bought other types of tests. But use of such direct-to-consumer medical tests varies greatly by age, race/ethnicity, marital status, income and years of education, according to the new report from the National Poll on Healthy Aging.
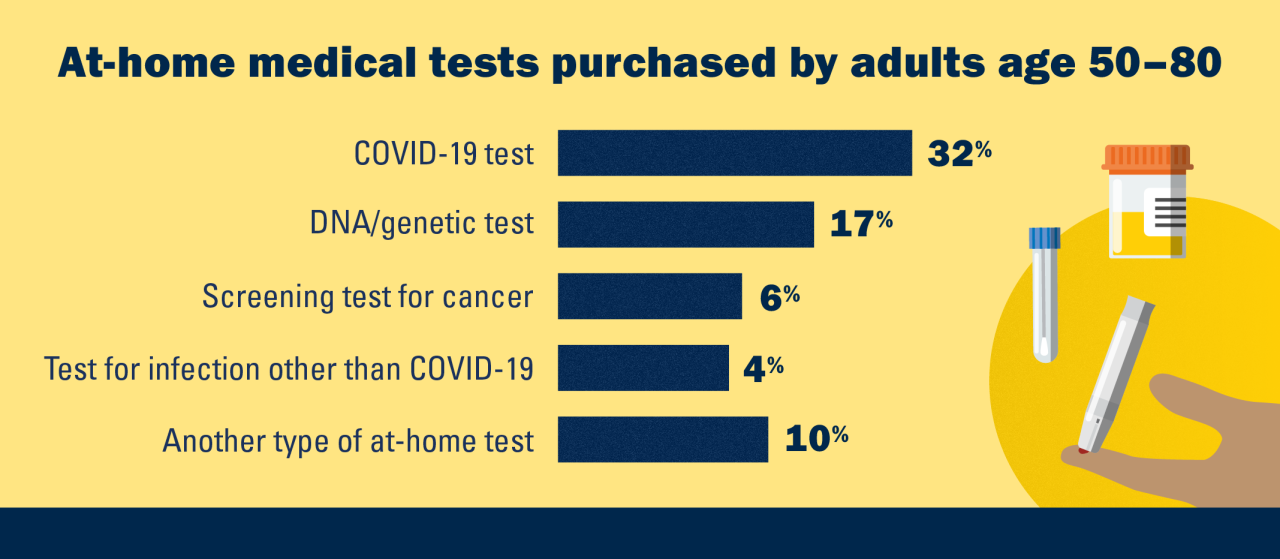
Even so, 82% of older adults say that in the future, they would be somewhat or very interested in taking a medical test at home.
The vast majority (92%) of older adults agree that the results they receive from these tests should be shared with the person’s doctor or other provider. But among those who actually have bought and used a home test for a non-COVID-19 infection such as HIV or a urinary tract infection, just 55% shared their result with their primary care provider, the poll shows. On the other hand, 90% of those who bought and used a cancer-related home test said they shared the result.
The poll is based at the U-M Institute for Healthcare Policy and Innovation and supported by AARP and Michigan Medicine, U-M’s academic medical center.
“As more companies bring these direct-to-consumer tests to market, and buy ads promoting them, it’s important for health care providers and policymakers to understand what patients might be purchasing, what they’re doing with the results, and how that fits into the broader clinical and regulatory picture,” says Jeffrey Kullgren, M.D., M.P.H., M.S., the poll’s director.
“As we have seen in COVID-19, it’s important to share results from a home test with a provider so that it can be used to guide your care and be counted in official statistics,” adds Kullgren, a primary care physician and health care researcher at Michigan Medicine and the VA Ann Arbor Healthcare System.
The poll shows that 53% of older adults believe at-home tests are regulated by the government. The reality is complicated.
Many types of tests that people can buy themselves to take at home, or that they take at home on the advice of a health professional, are reviewed by the U.S. Food and Drug Administration as medical devices, or overseen by the FDA’s program for testing laboratories that process samples sent to them. But not all of the tests that people can buy directly online or in a store are regulated in this way. For instance, tests marketed as “wellness” tests rather than ones used for diagnosis or to guide treatment are not regulated; neither are those with minimal risk.
The FDA has a searchable database of home tests it has approved based on evidence about their safety and accuracy, and a page about the COVID-19 at-home tests it has authorized under emergency conditions. It also offers more information about direct-to-consumer tests and home use tests that involve a health care professional. But not all tests get the full FDA review; the agency advises consumers to ask the vendor or health care provider about the status of a test.
“Home tests can be a convenient way for older adults to check if they have an illness, such as COVID-19” says Indira Venkat, Senior Vice President, AARP Research. “But consumers should make sure they know whether the test they are taking is FDA-approved, and how their health or genetic information might be shared.”
More about the poll findings:
Note: Respondents were asked to respond based on tests they had bought themselves online or at a store, not those given to them by a health care provider to collect a sample at home or those given to them for free.
- 6% of respondents had bought an at-home test for cancer, such as colon cancer or prostate cancer
- 4% had bought an at-home test for a non-COVID-19 infection, such as urinary tract infection or HIV
- 10% had bought another kind of at-home test, such as ones for allergies, food sensitivities or hormones, including those related to menopause or testosterone levels
- Black older adults were much less likely to have bought an at-home medical test than White or Hispanic older adults; this was true for COVID-19 tests (20% vs 33%) and non-COVID-19 tests (16% vs 30%)
- Purchasing of at-home COVID-19 tests was highest among older adults age 50 to 64 compared with those age 65 to 80. Purchasing of other types of tests did not differ by age group.
- Older adults were more likely to have bought at-home tests if they had more years of education, higher household incomes or are married.
- Advertising played a role in many older adults’ decisions to buy an at-home test, including 44% of those who took a DNA test and 11% of those who took a cancer test.
- 74% of older adults see at-home tests as more convenient than ones taken through their health care provider
- 59% agree that at-home tests can be trusted to provide reliable results
- Of the 82% who expressed interest in taking at-home tests in the future, the specific percentages were 70% for COVID-19 tests, 56% for cancer-related tests, and 43% for other types of infection. Interest was much higher among those who had used home tests before, and among women compared with men.
The poll report is based on findings from a nationally representative survey conducted by NORC at the University of Chicago for IHPI, and administered online and via phone in July 2022 among 2,163 adults age 50–80. The sample was subsequently weighted to reflect the U.S. population. Read past National Poll on Healthy Aging reports and about the poll methodology.
