Efficient testing during the COVID-19 pandemic continues to be a problem and a priority of public health leaders. This IHPI brief examines the current supply chain landscape of COVID-19 testing in Michigan, identifies key barriers to scale-up, and outlines policy considerations.
COVID-19 Testing Scale-Up: Key Issues and Considerations for Michigan Policymakers
 Efficient diagnostic testing continues to be a problem and a priority of public health leaders during the COVID-19 pandemic. While testing has scaled to 381 tests per 100K people per day in Michigan as of October 21, 2020 (seven-day average), many estimate that the current testing capacity and turnaround times are insufficient to keep viral transmission at bay. Multiple challenges remain to scale testing to a level that can effectively serve the volume and complexity of the issue at hand within an acceptable turnaround time.
Efficient diagnostic testing continues to be a problem and a priority of public health leaders during the COVID-19 pandemic. While testing has scaled to 381 tests per 100K people per day in Michigan as of October 21, 2020 (seven-day average), many estimate that the current testing capacity and turnaround times are insufficient to keep viral transmission at bay. Multiple challenges remain to scale testing to a level that can effectively serve the volume and complexity of the issue at hand within an acceptable turnaround time.
A research team at the University of Michigan assessed the situation from a process, supply chain, and governance perspective and identified key barriers to scale-up, providing policy options for leaders going forward. While these findings are focused on the state of Michigan, they are widely applicable to other states as well.
A multi-disciplinary team of clinical, public health, and business experts conducted more than 20 interviews with stakeholders both internal and external to the state of Michigan to gain diverse insights into this complex, multi-faceted issue.
The stakeholders interviewed included state leaders from the Michigan Department of Health and Human Services (MDHHS), public health experts, laboratory directors, epidemiologists, medical providers, manufacturers, data scientists, non-profit organizations, mathematicians, and purchasing cooperatives. Organizations consulted included the American Clinical Laboratory Association (ACLA), Association of Public Health Laboratories (APHL), Michigan Health & Hospital Association (MHA), The MITRE Corporation, MMCAP Infuse, National Association of State Procurement Officials (NASPO), National Governors Association (NGA), and Visiting Physicians Association (VPA).
Current Landscape of Diagnostic Testing
Diagnostic testing for active viral infection (reverse transcription polymerase chain reaction [RT-PCR], which tests for viral RNA) on nasopharyngeal (NP) specimens has been the gold standard for detection of COVID-19. Management of this testing landscape requires coordination of actions of many players who are influenced by information and material flows between them. To deal with high levels of uncertainty and a sense of urgency, organizations are constantly pursuing new approaches to increase testing capacity. These well-meaning approaches taken by various stakeholder groups, however, have resulted in fragmented information and material flows. This is further exacerbated by lack of clear network-wide visibility of the current landscape of testing, misaligned incentives, and unclear and shifting priorities. This makes overall coordination of the testing process difficult to orchestrate at the state level.
Key Findings from Our Stakeholder Interviews
1. Process and Supply Chain Flowcharts
We have created two flowcharts to better illustrate the current landscape of testing:
- Process Flow: Exhibit I (see Appendix) shows the different process steps and associated times in the diagnostic testing process, from seeking an appointment to sample collection to processing and reporting of results.
- The end-to-end diagnostic testing process flow time varies greatly due to a variety of factors, most important of which are the queuing times evident at each stage of the process.
- Variability and uncertainty in sample arrivals combined with the unpredictable availability of materials impact processing capacity in labs and lead to long turnaround times. Many experts have suggested an ideal turnaround time of two days or less; however, a national survey conducted by the Larremore Lab in July 2020 shows that average delays were closer to six days and up to 14 days or greater in some areas of the U.S.
- Long test turnaround times make it difficult for people to take the proper precautions while awaiting results and delay state and health department follow up actions, such as contact tracing.
- Supply Chain Flow: Both sample collection and processing require different types of materials (e.g., swabs, transport media, reagents, extraction tools). Exhibit II (see Appendix) illustrates the complex material and information flows between various actors in the supply chain to secure supplies needed for sample collection and testing. Manufacturers of these supplies and kits receive orders directly from labs, state governments, and the federal government. Labs may also place orders with their state governments who may request supplies from the federal stockpile.The pathways of information flow are complex, causing stakeholders to compete and creating challenges for manufacturers, who must interpret the unclear demand signals and develop their own prioritization methods.
2. Issues Influencing Capacity and Scale-up of Testing
![]() Labs
Labs
- Lack of visibility into the supply process — what, when, and how many supplies labs will get — hampers capacity planning
- Fragmentation of test platforms: Labs invest in multiple test platforms to increase capacity and diversify risk of supply shortages, which exacerbates the unpredictability of supply needs
- Lack of federal and state guidelines around how to prioritize testing of samples
- Technology challenges in capturing and reporting of test results, such as data cleansing efforts and manual uploading, lead to further delays
- Human resource and space constraints limit capacity expansion
- Lack of financial incentives (e.g., payments for test processing) that could further improve turnaround times
- Payments to labs differ by payer, creating a barrier to building a collaborative lab network to improve turnaround times
![]() Sample Collection Points
Sample Collection Points
- Shortage of supplies/PPE and quality concerns constrain capacity
- Staffing and training requirements slow ramp-up efforts
- Manual data collection processes pose challenges to accurate and timely reporting of results and follow up
![]() Manufacturers
Manufacturers
- Lack of clear or consistent demand signals from customers — how much, for how long, and when needed — affect production planning and allocations to ensure steady supply
- Fragmented demand signals due to multiple information flows regarding orders from federal, state and local governments, labs, and other entities cloud the view of the true demand
- Lack of guidance on prioritization necessary to inform supply allocation decisions
- Lack of a long-term strategic testing plan at the state and federal level with associated supply commitments prevents manufacturers from being able to assess needs and scale-up production capacity
- Federal regulatory barriers inhibit rapid innovation for testing and materials
![]() The General Public
The General Public
- Long test turnaround times increase anxiety and impede adherence to quarantine and isolation
- Limited access to testing sites, especially for vulnerable populations
- Unclear guidance on eligibility to get tested
- Uncertainty about whether costs of tests are covered
- Uncertainty introduced from publicity of ‘inaccurate’ tests
![]() Health Professionals
Health Professionals
- Unsure about how to prioritize who is tested, based on the patient's individual situation
- Unsure of which type of test should be used for which use-case
![]() State
State
- Lack of visibility into lab network capacity and congestion
- Imbalance of demand and supply/capacity across the lab network and inability to mitigate it
- Inefficient email and phone communication processes between labs and collection points
- Limited visibility into supplies available from the federal government and manufacturers
- Long turnaround times and inconsistent and incomplete reporting of test results by labs hampers containment and mitigation efforts (e.g., contact tracing and isolation), due to missing patient address and demographic information or duplicate results submitted by lab and provider, for example
- Incomplete performance measurement (e.g., on lab turnaround times) impedes optimal utilization of scarce assets
- Scarce resources pose a challenge to ensuring adequate access to testing, especially for vulnerable populations
- Little specific federal guidance for developing a segmented testing strategy (e.g., defining which types of tests are appropriate for surveillance vs. symptomatic outpatient testing) and prioritization within these categories, inhibiting optimal utilization of current capacity and additional capacity investment
- Insufficient volume of demand within a single state to incentivize capacity investment for high throughput labs
![]() Federal
Federal
- Insufficient investments in scale-up of testing capacity across the nation
- Inconsistent data reporting across states hampers the national response
- Incomplete performance measurement (e.g., on lab turnaround times) impedes optimal utilization of scarce assets
Key Considerations for Health Policy Decision Makers
The resulting policy options draw from research, interviews, and observations across stakeholder groups and cross-functional team discussions. They are also informed by industry best practices on resiliency and crisis response management across a wide spectrum of disruptions caused by H1N1, 2011 Japan Tsunami, etc. While the concepts we propose are focused on the state of Michigan, they are applicable across other states as well, and would do well to build upon existing structures to ensure long-term sustainability and evolution post-pandemic. It is important that policymakers weigh the implications of decisions across these four areas (visual management, incentives and investments, testing strategies, and governance and coordination) as part of a well-orchestrated rapid response to the pandemic.
1. Visual Management (e.g., using dashboards)
The use of internal dashboards that are updated in real-time could provide a quick overview of the current landscape of testing operations across the state and aid in efficiently and effectively managing the network of labs and sample collection points. Once essential key performance indicators are identified, standardized mechanisms to capture data from collection points and labs in real-time will be needed. This information can then be used by state leaders to address any current or potential future areas of concern.
Our team has provided illustrative examples of dashboards, along with metrics for consideration*:
- Figure I: Laboratory Capacity Assessment and
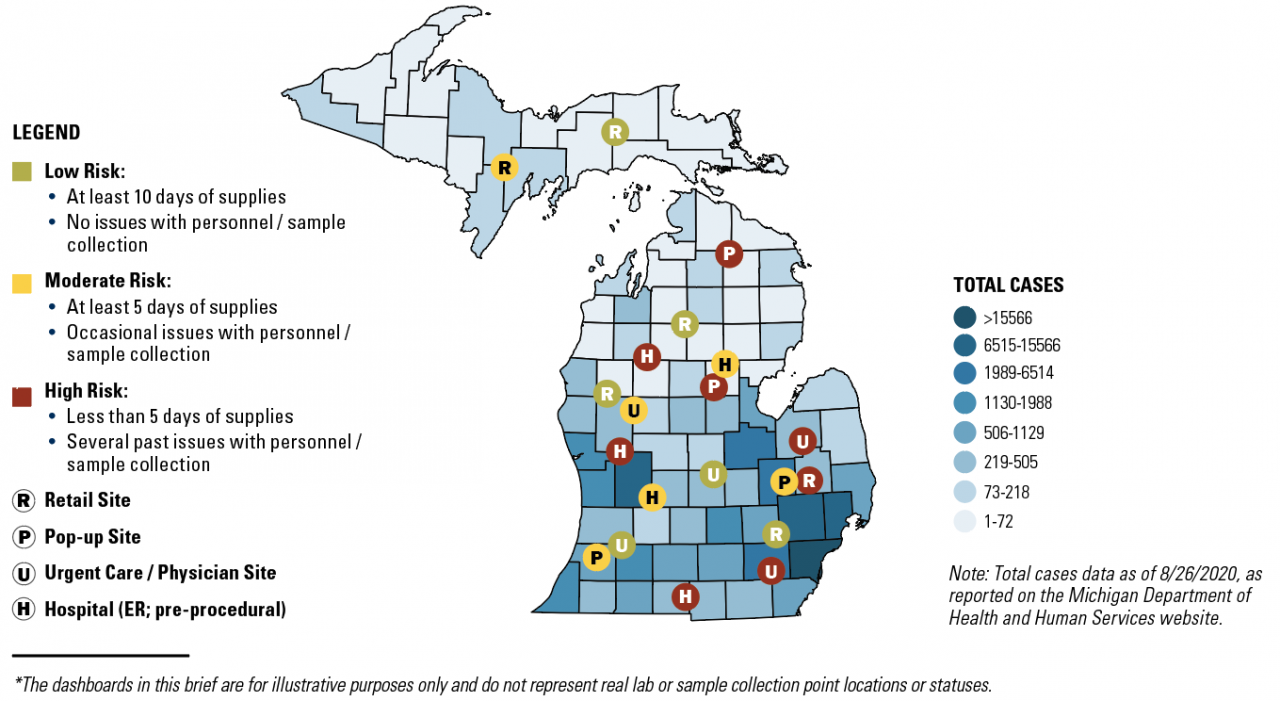
- Figure II: Sample Point Collection Assessment
These two dashboards show the locations of labs and sample collection points across the state, color coded by current status based on select metrics (low, moderate, or high risk) and overlaid on a map that shows the prevalence of disease.
- Exhibit III: Michigan Internal State Dashboard to Assess Progress (see the Appendix):
These dashboards can be used to track the progress of response management for scale-up of testing across the state. Areas tracked include testing demand, lab turnaround time, supply procurement progress, lab inventory tracking, sample collection point inventory tracking, lab needs assessment.
Figure I: Laboratory Capacity Assessment (illustrative)*
REAL-TIME STATUS
Data presently collected:
- Current number of tests performed per day
- Avg. test turnaround time
Additional data to capture, if possible:
- Current backlog of samples (function of turnaround time)
- Days of supplies to continue testing
- Severity and nature of current constraints (materials/staff/technology)
OTHER LAB DATA
- Lab primary contact and address
- Machine type(s) in use
- Sample types, swabs, and transport media accepted
- Current and maximum capacity (tests/day)
- Lab type (hospital/commercial/academic/public health)
- Lab proximity to sample collection sites
- Does integration exist between lab and state reporting system?
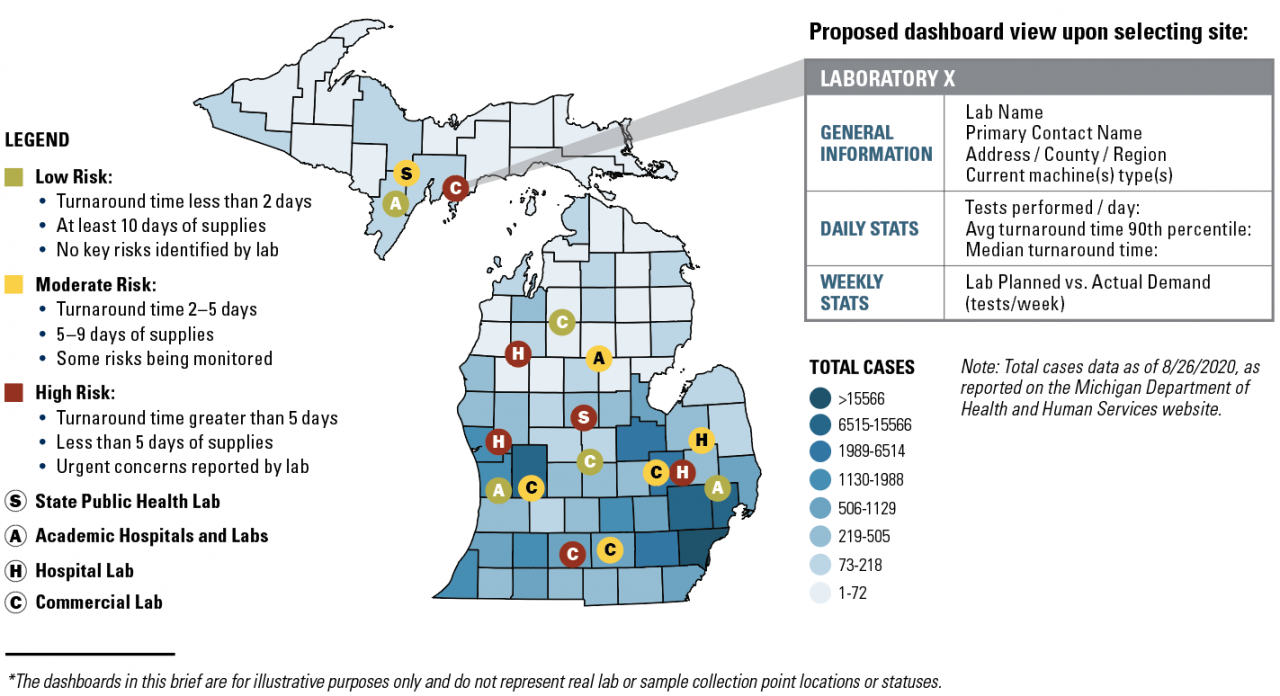
Figure II: Sample Collection Point Assessment (illustrative)*
Current Status for Each Site:
- Number of samples collected per day
- Days of supplies available to continue sample collections
- Severity and nature of current constraints (materials/staff)
State Level Data to Overlay:
- Population vulnerability index for that region
- Disease hot spot region
Other Collection Point Data:
- Site contact and location information
- Collection point type (short-term, long-term, retail vs. urgent care)
- Daily sample collection capacity
- Type of sample collected at site
- Any issues with sample collection (accuracy of data capture, etc.)
- What lab(s) do the samples go to?
- Type of staff collecting samples
2. Incentives & Investments
Scale-up of testing requires providing appropriate incentives to motivate various stakeholders to coordinate their actions, optimize existing capacity and make well-informed investments.
- Incentivize faster total turnaround time, from samples receipt to results reporting, by adjusting payment systems based on turnaround times achieved.
- Standardize payment mechanisms for COVID-19 tests to labs to mitigate incentive misalignment and optimize lab capacity utilization.
- Coordinate with other states to pool investments to incentivize testing capacity investment fit for purpose (e.g., recent compact including Michigan and five other states to purchase 3.5 million tests).
3. Testing Strategies
A pathway to returning to normal should include strategies to continue to expand testing capacity beyond symptomatic individuals through increasing the availability of different types of diagnostic tests.
- Develop a clear segmentation strategy to best match the purpose of the test (e.g., diagnostic, screening, surveillance) with the type of tests (e.g., RT-PCR, Rapid Antigen Tests, Serologic/Antibody tests).
- Develop prioritization criteria for RT-PCR diagnostic tests to reduce turnaround times.
- Develop appropriate mechanisms to ensure implementation of the prioritization strategy and traceability to ensure compliance.
- Develop smart pooling methodologies to reduce the volume of processing that a lab needs to conduct, therefore improving capacity utilization.
- Leverage private sector personnel expertise as needed in order to help manage challenges of procurement and supply chain management.
4. Governance & Coordination
Effective and strategic coordination and steering of multiple stakeholders’ mitigation and scale-up strategies requires an architecture to orchestrate design, planning, and execution of the state level response.
State leaders could consider developing tracks (or working groups) to help oversee the testing landscape on the state level. Exhibit IV lists possible tracks with potential participants, descriptions, and illustrative outputs to aid in mitigation planning and execution strategies.
As appropriate, the tracks may use dashboards (such as Figures I and II, Exhibit III) to monitor the current testing landscape and respond to any emerging issues. Tracks should report their progress and pressing issues to state leadership as needed.
REFERENCES
A Better Way to Scale COVID-19 Testing.
Bhojwani N, Gawande A. Harvard Business Review. 2020 Jul 7. https://hbr.org/2020/07/a-better-way-to-scale-covid-19-testing
Cisco SCRM in Action: 2011 Tohoku Earthquake Teaching Case.
William Davidson Institute Publishing. 2013 Mar 6. https://wdi-publishing.com/product/cisco-scrm-in-action-2011-tohoku-ear…
Cisco SCRM in Action: 2011 Tohoku Earthquake Teaching Case: Video Interview with Case Protagonist.
https://www.youtube.com/watch?v=-xvYaMKmBSQ&feature=youtu.be.
Coronavirus Michigan Data.
https://www.michigan.gov/coronavirus/0,9753,7-406-98163_98173---,00.html. Accessed August 26, 2020.
COVID-19 Rapid Response Impact Initiative, White Paper 9v2: The Mechanics of the COVID-19 Testing Supply Chain.
Allen DS, Weyl EG, Guthrie KM. Edmond J. Safra Center for Ethics, Harvard University. 2020 Apr 22. https://ethics.harvard.edu/files/center-for-ethics/files/9v2testingsupp…
COVID-19 Rapid Response Impact Initiative, White Paper 14: Designing an Interstate Compact for a Pandemic Testing Board. Hansmann L, Sitaraman G. Edmond J. Safra Center for Ethics, Harvard University. 2020 May 4. https://ethics.harvard.edu/files/center-for-ethics/files/wp14designingi…
COVID Test Delays.
COVID-19 Testing Group. 2020. https://larremorelab.github.io/covid19testgroup. Accessed August 26, 2020.
Governors of Six States Announce Major Bipartisan Compact for Three Million Rapid Antigen Tests.
The Rockefeller Foundation. 2020 Aug 4. https://www.rockefellerfoundation.org/news/governors-of-six-states-anno… August 26, 2020.
MI Safe Start Map.
2020. https://mistartmap.info. Accessed September 20, 2020.
National COVID-19 Testing Action Plan: Pragmatic Steps to Reopen Our Workplaces and Our Communities.
The Rockefeller Foundation. 2020. https://www.rockefellerfoundation.org/national-covid-19-testing-action-….
Testing Sensitivity is Secondary to Frequency and Turnaround Time for COVID-19 Surveillance.
Larremore DB, Wilder B, Lester E, et al. MedRxiv (preprint). 2020 Jun 27. PMID: 32607516. doi:10.1101/2020.06.22.20136309.
Tracking COVID-19 in the United States: From Information Catastrophe to Empowered Communities.
Resolve to Save Lives, Prevent Epidemics project. 2020 Jul 21. https://preventepidemics.org/wp-content/uploads/2020/07/RTSL_Tracking-C…
AUTHORS
Ravi Anupindi, PhD, ME, MS, Ross School of Business, University of Michigan
Lee Schroeder, MD, PhD, Department of Pathology, University of Michigan
Rajan Dewar, PhD, MBBS, Department of Pathology, University of Michigan
Surabhi Rajaram, MPH, Bill & Melinda Gates Foundation
Emily Edkins, MBA (expected 2021), Ross School of Business, University of Michigan
ACKNOWLEDGMENTS
This policy brief was supported by the IHPI Policy Sprint program, which provides funding and staff assistance to IHPI member-led teams in undertaking rapid analyses to address important health policy questions and develop products that inform decision-making at the local, state, or national level.
FOR MORE INFORMATION
Please contact Eileen Kostanecki, IHPI’s Director of Policy Engagement & External Relations, at [email protected].
